Vaccine Adjuvants Market Size to Reach USD 3,933.7 Mn in 2032 – Rise with Steller CAGR 13.6% | Market.us
Page Contents
Market Overview
Published Via 11Press : Vaccine adjuvants market are substances that enhance the body's immune response to a vaccine. They are added to vaccines to increase their effectiveness and provide long-lasting protection against specific diseases. Adjuvants work by stimulating the immune system, causing it to produce more antibodies and activate other immune cells that can recognize and attack the targeted pathogen.
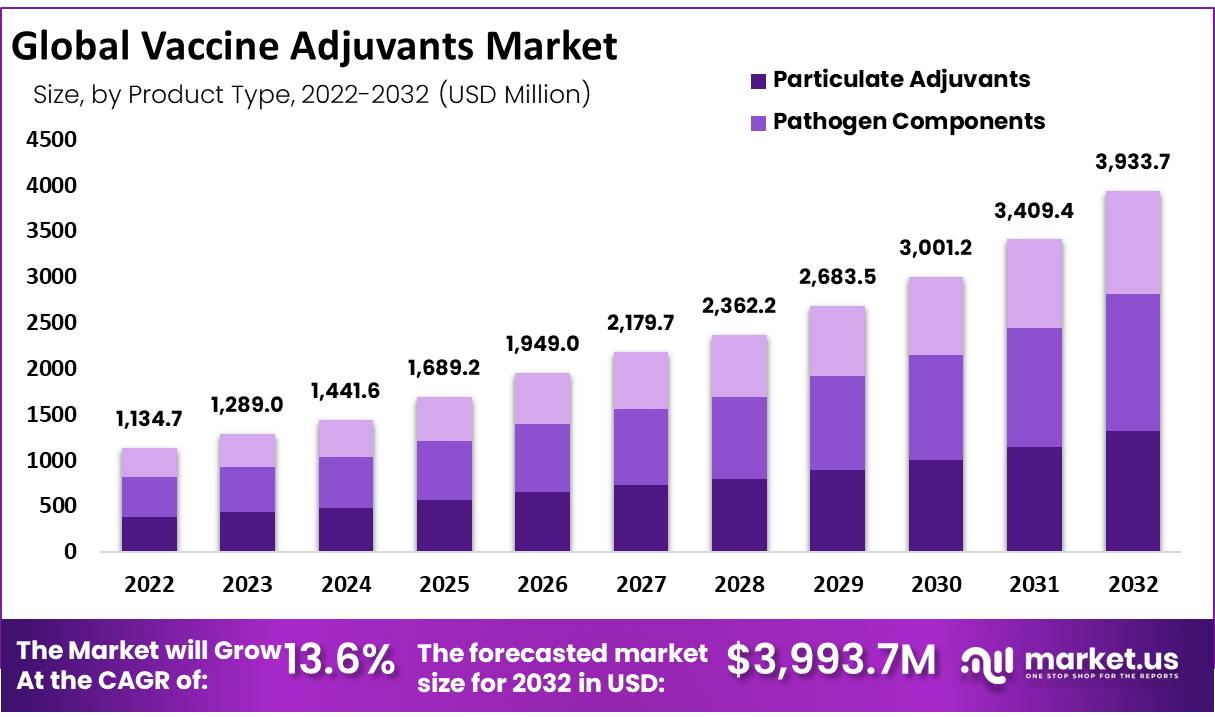
The global vaccine adjuvants market size is expected to be worth around USD 1,134.7 Million by 2022 from USD 3,933.7 Million in 2032, growing at a CAGR of 13.6% during the forecast period from 2022 to 2032.
There are several types of vaccine adjuvants, including aluminum salts, oil-based emulsions, liposomes, and virosomes. Aluminum salts are the most commonly used adjuvant in vaccines because they have been shown to be safe and effective in improving vaccine efficacy. Oil-based emulsions such as MF59 and AS03 have also been approved for use in some influenza vaccines.
The use of adjuvants is particularly important in developing vaccines for diseases that are difficult to prevent or treat with traditional methods. For example, adjuvanted influenza vaccines have been shown to be more effective than non-adjuvanted vaccines in protecting older adults whose immune systems may not respond as well to vaccination. The development of new adjuvant technologies is an active area of research aimed at improving vaccine effectiveness and reducing the number of doses needed for optimal protection against infectious diseases.
Key Takeaway
- by Product Type 2022. The particulate segment is expected to earn a revenue portion of 34.4% by 2022.
- Through the Route of Administration, the research sector dominated by growth rate over the 2023-2032 forecast timeframe. 2032.
- Through Application, the research sector dominates the market and is expanding by a CAGR of 7.8 percent in the timeframe 2023-2032.
- By 2022 North America led the market, with the largest share of revenue of 39%.
- Europe was able to record a 22.4 percent revenue share by 2022.
- Asia-Pacific will expand by 5.2 percent between 2023 and 2032.
Request For Sample Report Here: https://market.us/report/vaccine-adjuvants-market/request-sample/
Regional Snapshot
- North America:
- It is believed that the North American market has been an essential player in the adjuvants for the vaccines sector due to the presence of established pharmaceutical firms and research institutes.
- The United States is the largest market in the world that is driven by a robust health system, a high level of investments in development and research, and a large population of consumers.
- Canada is also playing a major contribution to the global market in particular, with an emphasis on the production and development of vaccines.
- Europe:
- Europe is home to a highly developed healthcare system with an emphasis on vaccination programs. This is a major reason for the increased demand for adjuvants to vaccines.
- countries like Germany and Italy, Germany United Kingdom, France, and Italy are major market players within the region.
- It is the European market is marked by the presence of prominent manufacturers of vaccines and a favorable regulatory system.
- Asia Pacific:
- The Asia Pacific region is expected to see rapid growth in the market for vaccine adjuvants due to increased expenditures in healthcare infrastructure, the growing population and increasing awareness of the importance of vaccination.
- Countries like China, India, Japan, and South Korea are driving the expansion of the market in this region.
- The existence of contract manufacturing companies (CMOs) in nations such as India as well as China has also contributed to the development of adjuvants' manufacturing capabilities.
- Latin America:
- Latin America has shown significant improvements in the use of vaccine adjuvants which is primarily due to the government's initiatives to upgrade the infrastructure for healthcare and boost the rate of vaccination.
- Brazil, Mexico, and Argentina are the major regional markets and are focusing more on the development of vaccines and production.
- Middle East and Africa:
- The market for adjuvants to vaccines within the Middle East and Africa is growing at a steady pace because of increased investment in health infrastructure as well as strategies to tackle infectious diseases.
- South Africa, Saudi Arabia as well as Saudi Arabia, as well as the United Arab Emirates are the principal contributors to the markets in the region.
Drivers
- Increasing Vaccination Programs: The growing emphasis on vaccination programs worldwide, especially for diseases like influenza, hepatitis, and human papillomavirus (HPV), is driving the demand for vaccine adjuvants. Adjuvants help enhance the immune response, leading to improved vaccine efficacy and broader protection.
- Rising Prevalence of Infectious Diseases: The global burden of infectious diseases, including viral and bacterial infections, is a significant driver for the vaccine adjuvants market. Adjuvanted vaccines can provide better protection against these diseases, leading to increased demand.
- Technological Advancements: Advances in adjuvant technologies have expanded the range of applications in vaccine development. Novel adjuvants are being developed to improve vaccine safety, increase immunogenicity, and enable the development of vaccines for challenging pathogens.
- Government Initiatives and Funding: Governments worldwide are actively promoting vaccination campaigns and investing in research and development activities related to vaccines. Supportive government policies, grants, and funding are driving the growth of the vaccine adjuvants market.
- Growing Aging Population: The elderly population is more susceptible to infectious diseases, making them a high-priority group for vaccination. Vaccine adjuvants help enhance the immune response in older individuals, leading to improved protection against diseases.
Restraints
- Stringent Regulatory Requirements: Adjuvants are subject to stringent regulatory requirements to ensure their safety and efficacy. The complex regulatory landscape can pose challenges for market players in terms of compliance and approval processes, leading to delays and increased costs.
- Vaccine Hesitancy and Safety Concerns: Vaccine hesitancy, fueled by misinformation and safety concerns, can affect the uptake of vaccines, including adjuvanted vaccines. Public perception and confidence in vaccination can impact the demand for vaccine adjuvants.
- High Development Costs: Developing adjuvanted vaccines involves significant research and development costs, including clinical trials and regulatory compliance. The high cost of development can be a barrier, particularly for small and medium-sized enterprises (SMEs) in the market.
- Limited Adoption in Developing Countries: The adoption of adjuvanted vaccines in developing countries may be limited due to cost constraints, lack of infrastructure, and challenges in vaccine distribution and storage, affecting the demand for vaccine adjuvants.
- Adverse Reactions and Side Effects: Although vaccine adjuvants are generally considered safe, there can be rare instances of adverse reactions or side effects. Safety concerns related to adjuvanted vaccines can influence public perception and acceptance of these products.
Opportunities
- Increasing Demand for Next-Generation Vaccines: The development of next-generation vaccines, such as mRNA-based vaccines and recombinant subunit vaccines, presents opportunities for the use of adjuvants. Adjuvants can play a crucial role in enhancing the efficacy of these new vaccine platforms.
- Emerging Infectious Diseases: The ongoing threat of emerging infectious diseases, such as Zika virus, Ebola, and COVID-19, provides opportunities for the vaccine adjuvants market. Adjuvanted vaccines can help in the rapid development of effective vaccines against these novel pathogens.
- Expansion of Immunization Programs: Many developing countries are expanding their immunization programs to address the burden of infectious diseases. This presents an opportunity for the adoption of adjuvanted vaccines and the growth of the vaccine adjuvants market in these regions.
- Advancements in Adjuvant Technologies: Continuous advancements in adjuvant technologies, such as the development of novel adjuvants with improved safety profiles and targeted immune responses, create opportunities for market players to introduce innovative adjuvanted vaccines.
- Collaborations and Partnerships: Collaborations between pharmaceutical companies, research institutions, and government organizations can facilitate the development and commercialization of adjuvanted vaccines. Strategic partnerships can help overcome challenges and unlock opportunities in the market.
Make an inquiry before picking up this report @ https://market.us/report/vaccine-adjuvants-market/#inquiry
Challenges
- Safety and Regulatory Concerns: The safety and regulatory requirements for adjuvanted vaccines can pose challenges for market players. Demonstrating the safety and efficacy of adjuvanted vaccines, complying with regulatory standards, and addressing public concerns are key challenges in the market.
- Vaccine Hesitancy and Misinformation: Vaccine hesitancy fueled by misinformation and concerns about vaccine safety can hinder the acceptance and uptake of adjuvanted vaccines. Addressing vaccine hesitancy through education and communication is crucial for market growth.
- Manufacturing and Scale-up Challenges: Manufacturing adjuvanted vaccines at a large scale can be challenging due to process complexities, cost considerations, and the need for specialized facilities. Developing efficient manufacturing processes and ensuring a stable supply chain are key challenges in the market.
- High Development Costs: The development of adjuvanted vaccines involves substantial research and development costs, including clinical trials and regulatory compliance. The high cost of development can be a barrier, particularly for smaller companies and organizations with limited financial resources.
- Intellectual Property Rights: Intellectual property rights and patent-related issues can impact the competitive landscape of the vaccine adjuvants market. Patent disputes and the availability of generic adjuvants can affect market dynamics and profitability for market players.
Key Market Players
- Merck KGaA
- Dynavax Technologies Corporation
- Croda International Plc
- Novavax, Inc.
- CSL Limited
- AstraZeneca plc
- MPV Technologies LLC
- Adjuvance Technologies, Inc.
- SPI Pharma
- Agenus Inc.
- Astellas Pharma, Inc.
- CureVac N.V.
- Sanofi S.A.
- GlaxoSmithKline plc
- OZ Biosciences
- Vaxine Pty Ltd
- Merck & Co, Inc.
- Phibro Animal Health Corporation
- Brenntag Biosector
- Brenntag SE
- Other Key Players
Key Market Segments
By Product Type
- Adjuvant Emulsion
- Particulate Adjuvants
- Pathogen Components
By Route of Administration
- Oral
- Subcutaneous
- Intramuscular
- Intradermal
- Intranasal
By Application
- Research
- Commercial
Top Impacting Factors
- Disease Prevalence and Outbreaks: The prevalence of infectious diseases and outbreaks play a crucial role in driving the demand for vaccines and, consequently, vaccine adjuvants. The occurrence of epidemics or pandemics can lead to a surge in vaccine development activities and the need for adjuvants to enhance vaccine efficacy.
- Government Immunization Policies: Government immunization policies and initiatives have a direct impact on the vaccine adjuvants market. The introduction of new vaccines into national immunization programs, expansion of vaccination coverage, and funding for vaccine development and production can significantly drive the demand for adjuvanted vaccines.
- Technological Advancements: Advancements in vaccine technologies and adjuvant formulations have a profound impact on the market. Innovative adjuvant technologies that offer improved safety, increased immunogenicity, and better antigen presentation can drive the adoption of adjuvant vaccines.
- Regulatory Landscape: Regulatory frameworks and guidelines play a critical role in shaping the vaccine adjuvants market. Stringent regulatory requirements for the approval and commercialization of adjuvanted vaccines can impact the time and cost of development, as well as market entry barriers for new players.
- Vaccine Hesitancy and Public Perception: Vaccine hesitancy, driven by factors such as safety concerns, misinformation, and distrust, can affect the demand for adjuvant vaccines. Public perception and acceptance of vaccines, including those containing adjuvants, play a significant role in the market's growth.
- Healthcare Infrastructure and Access: The availability and accessibility of healthcare infrastructure, including vaccination centers, cold chain storage, and distribution networks, are crucial factors that influence the adoption of adjuvant vaccines. Adequate infrastructure is necessary for the effective utilization of vaccines and adjuvants.
Recent Developments
- February 2022 –As the base of their applications for regulatory approval for the COVID-19 vaccine, Sanofi and GSK aim to present results from their promoter and clinical trials in Phase 3.
- September 2021Novavax Inc. has enrolled the very first participant in the Phase 1/2 trial, which is evaluating the efficacy and safety of vaccines that combine Novavax influenza as well as COVID-19. The clinical trials will include recombinant protein-based NanoFlu candidate and patent-pending saponin-based Matrix – M adjuvants in a single formulation.
Report Scope
| Report Attribute | Details |
| The market size value in 2022 | USD 1,134.7 Mn |
| Revenue Forecast by 2032 | USD 3,933.7 Mn |
| Growth Rate | CAGR Of 13.6% |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, and Middle East & Africa, and Rest of the World |
| Historical Years | 2017-2022 |
| Base Year | 2022 |
| Estimated Year | 2023 |
| Short-Term Projection Year | 2028 |
| Long-Term Projected Year | 2032 |
Frequently Asked Questions
Q: What is the current size of the Vaccine Adjuvants Market?
A: The Global Vaccine Adjuvants Market size is USD 1,134.7 Million in 2022.
Q: What is the projected growth rate for Vaccine Adjuvants Market?
A: The Vaccine Adjuvants Market is expected to grow at a CAGR of 13.6% from 2023 to 2032.
Q: What are some of the key players in the Vaccine Adjuvants Market?
A: Some of the key players in the Vaccine Adjuvants market include Merck KGaA, Dynavax Technologies Corporation, Croda International Plc, Novavax, Inc., CSL Limited, AstraZeneca plc, MPV Technologies LLC, Adjuvance Technologies, Inc., SPI Pharma, Agenus Inc., Astellas Pharma, Inc., CureVac N.V., Sanofi S.A., GlaxoSmithKline plc, OZ Biosciences, Vaxine Pty Ltd, Merck & Co, Inc., Phibro Animal Health Corporation, Brenntag Biosector, Brenntag SE, Other Key Players
Contact
Global Business Development Team – Market.us
Market.us (Powered by Prudour Pvt. Ltd.)
Send Email: [email protected]
Address: 420 Lexington Avenue, Suite 300 New York City, NY 10170, United States
Tel: +1 718 618 4351
Website: https://market.us
Content has been published via 11press. for more details please contact at [email protected]
The team behind market.us, marketresearch.biz, market.biz and more. Our purpose is to keep our customers ahead of the game with regard to the markets. They may fluctuate up or down, but we will help you to stay ahead of the curve in these market fluctuations. Our consistent growth and ability to deliver in-depth analyses and market insight has engaged genuine market players. They have faith in us to offer the data and information they require to make balanced and decisive marketing decisions.