Cell and Gene Therapy Market To Reflect Tremendous Growth Potential With A CAGR Of 22.6% BY 2032 | Market.us
Page Contents
Market Overview
Published Via 11Press : The cell and gene therapy market is a rapidly growing area of the healthcare industry that focuses on developing treatments for diseases through genetic modification. The market has grown significantly in recent years due to advancements in technology, increased investment from pharmaceutical companies, and a better understanding of how cells and genes work.
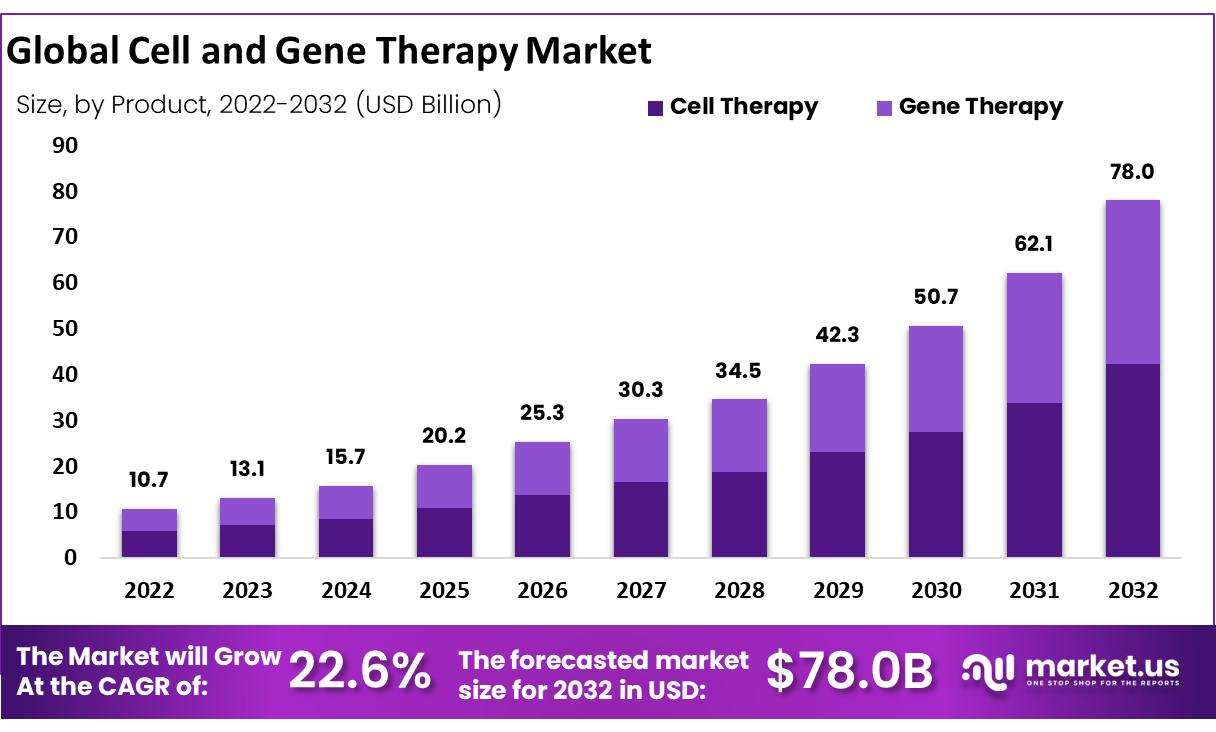
The global cell and gene therapy market size is expected to be worth around USD 10.7 Billion by 2022 from USD 78.0 Billion in 2032, growing at a CAGR of 22.6% during the forecast period from 2022 to 2032.
Currently, there are over 1,000 clinical trials underway globally for cell and gene therapies targeting various diseases such as cancer, genetic disorders, autoimmune diseases, and more. Overall, the cell and gene therapy market holds great promise for patients suffering from currently incurable diseases.
Request For Sample Report Here: https://market.us/report/cell-and-gene-therapy-market/request-sample/
Key Takeaway
- The cell therapy segment accounted revenue share of 47.6% in 2022.
- By therapy type, the gene therapy segment held a revenue share of 52.6% in 2022.
- The North American region has generated a revenue share of around 49.7% in 2022.
Exploring the Impact of Generative AI on the Cell and Gene Therapy Market
Generative AI is a rapidly developing field with the potential to revolutionize the cell and gene therapy market. By using machine learning algorithms to create new, original content, generative AI can be used to improve the design, development, and delivery of cell and gene therapies.
Design
One of the most promising applications of generative AI in cell and gene therapy is the design of new therapies. Generative AI can be used to identify new targets for therapy, design new vectors for delivering therapies, and optimize the dosing of therapies. For example, generative AI has been used to design new zinc finger proteins that can be used to turn genes on or off.
Development
Generative AI can also be used to improve the development of cell and gene therapies. Generative AI can be used to automate tasks that are currently performed manually, such as screening cells for mutations or optimizing the culture conditions for cells. This can help to reduce the time and cost of developing new therapies.
Delivery
Generative AI can also be used to improve the delivery of cell and gene therapies. Generative AI can be used to design new delivery vehicles for therapies, such as nanoparticles or liposomes. This can help to improve the efficacy and safety of therapies.
The Future of Generative AI in Cell and gene therapy
- The potential applications of generative AI in cell and gene therapy are vast. As technology continues to develop, we can expect to see even more innovative and effective ways to use generative AI to improve the lives of patients. In the future, generative AI could be used to:
- Design new therapies for a wider range of diseases
- Develop therapies that are more effective and safer
- Deliver therapies more efficiently and effectively
- Personalize therapies for each patient
Regional Snapshot
- North America: North America and especially its leading nation of the US has long been considered at the vanguard of gene and cell therapy markets, boasting robust regulatory structures, advanced healthcare infrastructure, substantial research funding sources, and key players operating within it. Furthermore, many clinical trials and recognized treatments can be found within North America itself with new funds coming from both public and private sectors to support commercialization of gene therapy/cell therapy developments and advances.
- Europe: Europe boasts a robust cell and gene therapy market with nations including Germany, France, the UK, and Switzerland holding key positions. The European Medicines Agency (EMA) actively regulates and approves gene and cell therapy use; with an active scientific and academic community and strong collaborations among academic industries healthcare providers suppliers among others in support. Furthermore, there are ongoing clinical trials as well as approved treatments focusing on rare genetic disorders as well as cancer.
- Asia-Pacific: The Asia-Pacific region is witnessing the remarkable expansion of gene and cell therapy markets. States including China, Japan, South Korea as well as Australia have invested significantly in this space; China in particular stands out due to an environment supportive of regulatory reform as well as having an expansive patient pool. Growing middle-class populations coupled with rising healthcare expenses as well as improvements to infrastructure have also contributed to an upsurge in demand for gene therapy/cell treatments in this part of Asia-Pacific.
- Latin America: Latin America has emerged as an attractive market for gene and cell treatments. Brazil, Mexico, and Argentina are major players in this region where clinical trials and breakthroughs in therapeutics are becoming more frequent. With cancer rates continuing to climb and expanding healthcare infrastructure driving demand for genetic treatments like gene therapy therapies in this part of Latin America – but with issues surrounding regulations, reimbursement mechanisms, accessing treatment as well as compliance are obstacles that must be resolved in order to maintain growth within this market place.
- Middle East and Africa: Gene and cell therapy markets within the Middle East and Africa are rapidly growing. Countries such as Israel and United Arab Emirates (UAE) are at the forefront of studies as well as clinical trials related to these therapies, while healthcare infrastructure, as well as regulatory frameworks in these regions, are adapting to accommodate cell and gene treatments; yet challenges related to access, affordability, and understanding must also be overcome to foster wider acceptance of such treatments.
Directly Purchase a copy of the report | Quick Delivery Available – buy: https://market.us/purchase-report/?report_id=101909
Drivers
- Advancements in Biotechnology: Rapid advancements in biotechnology, including gene editing tools like CRISPR-Cas9, have expanded the possibilities for developing and refining cell and gene therapies, driving innovation in the field.
- Increasing Prevalence of Genetic Disorders and Cancer: The rising incidence of genetic disorders and cancer has created a significant unmet medical need, increasing the demand for cell and gene therapies as potential curative or disease-modifying treatments.
- Favorable Regulatory Environment: Regulatory agencies in various regions have implemented expedited approval pathways and designated regulatory frameworks for cell and gene therapies, facilitating the development and commercialization process.
- Growing Investments and Funding: Increasing investments from both public and private sectors, as well as venture capital firms, are fueling research and development activities in the cell and gene therapy market.
- Technological Advancements: Advances in gene delivery systems, genetic engineering techniques, and manufacturing processes have improved the safety, efficacy, and scalability of cell and gene therapies, driving their adoption.
Restraints
- High Costs: The high costs associated with cell and gene therapies, including research and development, manufacturing, and patient-specific treatments, pose a significant challenge to widespread adoption and affordability.
- Manufacturing Complexities: The complex and personalized nature of cell and gene therapies presents manufacturing challenges, including scalability, quality control, and logistics, which can limit their widespread availability.
- Limited Clinical Evidence: Despite the promising potential of cell and gene therapies, there is still a need for long-term clinical data to establish their long-term safety, durability, and efficacy across a broader range of indications.
- Regulatory and Reimbursement Barriers: Despite the favorable regulatory environment, navigating the regulatory approval process and securing reimbursement can be challenging due to the unique characteristics of cell and gene therapies.
- Ethical and Social Considerations: The ethical implications of gene editing, genetic modifications, and the potential misuse of these technologies raise societal concerns that may influence public acceptance and regulatory decision-making.
Opportunities
- Expansion to New Indications: Cell and gene therapies have the potential to address a wide range of diseases beyond genetic disorders and cancer, including cardiovascular diseases, neurological disorders, and autoimmune diseases, providing significant opportunities for market expansion.
- Global Market Growth: The increasing adoption of cell and gene therapies in emerging markets, such as China, India, and Brazil, presents significant growth opportunities, driven by a large patient population and supportive healthcare policies.
- Personalized Medicine and Precision Therapeutics: Cell and gene therapies are at the forefront of personalized medicine, offering tailored treatments based on an individual's genetic profile, which can lead to better treatment outcomes and patient care.
- Collaborations and Partnerships: Collaboration between academia, industry, and healthcare providers can foster innovation, enhance research capabilities, and accelerate the development of cell and gene therapies.
- Patient Advocacy and Education: Increased patient advocacy and education initiatives can raise awareness, improve understanding, and foster acceptance of cell and gene therapies among patients, healthcare providers, and the general public.
Challenges
- Safety and Long-Term Efficacy: Ensuring the long-term safety and durability of cell and gene therapies, as well as managing potential off-target effects and immunological responses, remains a significant challenge.
- Manufacturing Scalability and Standardization: Developing scalable manufacturing processes and establishing standardized quality control measures are crucial for the wider availability and commercialization of cell and gene therapies.
- Supply Chain and Logistics: The complex nature of cell and gene therapies, including the need for cryopreservation, temperature-sensitive handling, and timely delivery, presents logistical challenges that need to be addressed.
- Intellectual Property and Patent Issues: Intellectual property disputes and challenges related to patent protection and licensing agreements can impact the development, commercialization, and market access of cell and gene therapies.
- Education and Training: The successful integration of cell and gene therapies into clinical practice requires training and education for healthcare providers to ensure proper patient selection, administration, and management of potential adverse events.
Market Players
- GalaxoSmithKline plc
- Novartis AG
- Amgen Inc.
- Bristol-Myers Squibb Company
- Spark Therapeutics
- Pfizer Inc.
- Biogen Inc.
- Thermo Fisher Scientific Inc.
- Other Key Players
Market Segmentation
By Therapy Type
- Cell Therapy
- Gene Therapy
By Indication
- Genetic Disorders
- Cardiovascular Disorders
- Neurological Disorders
- Oncological Disorders
- Other Indications
By End-User
- Hospitals
- Academic & Research Institutes
- Cancer Care Centers
- Other End-Users
Make an inquiry before picking up this report @ https://market.us/report/cell-and-gene-therapy-market/#inquiry
Top Impacting Factors
- Regulatory Environment: Favorable regulatory frameworks and expedited approval pathways for cell and gene therapies play a crucial role in driving their development, commercialization, and market access. Regulatory agencies' clear guidelines and support facilitate the translation of promising research into approved therapies.
- Clinical Success and Efficacy: The clinical success and demonstrated efficacy of cell and gene therapies in treating specific diseases or conditions significantly impact market growth. Positive clinical trial results and real-world evidence validate the therapeutic potential of these therapies and drive adoption.
- Safety Profile: The safety profile of cell and gene therapies is a critical factor influencing market acceptance and adoption. Ensuring the safety of patients, managing potential adverse events, and addressing long-term safety concerns is essential for building trust and confidence in these therapies.
- Market Access and Reimbursement: The availability of reimbursement and adequate market access significantly impact the adoption and commercial success of cell and gene therapies. Obtaining reimbursement approvals and demonstrating cost-effectiveness are important considerations for market penetration.
- Intellectual Property Rights: The protection of intellectual property rights and patents plays a vital role in incentivizing research and development, attracting investments, and promoting innovation in the field of cell and gene therapy.
Recent Developments
- October 2022-Pfizer Inc. introduced that they have successfully purchased Biohaven Pharmaceuticals which is a migraine drug manufacturing corporation. By Pfizers’acquistions, they will be capable to prevent migraine patients with more treatment substitutes.
- January 2021-Thermo Fisher Scientific Inc. achieved Henogen S.S. Novasep’s viral vector manufacturing business in Belgium, for approximately in millions. This achievement will rise its presence in the market.
- February 2020- Catalent Inc. introduced the achievement of MaSTher Cell Global, Inc., to strengthen its market portfolio in the cell and gene therapy market.
Report Scope
| Report Attribute | Details |
| The market size value in 2022 | USD 10.7 Bn |
| Revenue Forecast by 2032 | USD 78.0 Bn |
| Growth Rate | CAGR Of 22.6% |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, and Middle East & Africa, and Rest of the World |
| Historical Years | 2017-2022 |
| Base Year | 2022 |
| Estimated Year | 2023 |
| Short-Term Projection Year | 2028 |
| Long-Term Projected Year | 2032 |
Frequently Asked Questions
Q: What is the current size of the Cell And Gene Therapy Market?
A: The Global Cell And Gene Therapy Market size is USD 10.7 Bn in 2022.
Q: What is the projected growth rate for Cell And Gene Therapy Market?
A: The Cell And Gene Therapy Market is expected to grow at a CAGR of 22.6% from 2023 to 2032.
Q: What are some of the key players in the Cell And Gene Therapy Market?
A: Some of the key players in the Cell And Gene Therapy market include GlaxoSmithKline plc, Novartis AG, Amgen Inc., Bristol-Myers Squibb Company, Spark Therapeutics, Pfizer Inc., Biogen Inc., Thermo Fisher Scientific Inc., Other Key Players.
Contact:
Global Business Development Team – Market.us
Market.us (Powered by Prudour Pvt. Ltd.)
Send Email: [email protected]
Address: 420 Lexington Avenue, Suite 300 New York City, NY 10170, United States
Tel: +1 718 618 4351
Website: https://market.us/
Content has been published via 11press. for more details please contact at [email protected]
The team behind market.us, marketresearch.biz, market.biz and more. Our purpose is to keep our customers ahead of the game with regard to the markets. They may fluctuate up or down, but we will help you to stay ahead of the curve in these market fluctuations. Our consistent growth and ability to deliver in-depth analyses and market insight has engaged genuine market players. They have faith in us to offer the data and information they require to make balanced and decisive marketing decisions.