eClinical Solutions Market Size (USD 27 Billion by 2032) with 12.8% CAGR | According To Market.us

Page Contents
Market Overview
Published Via 11Press : The eClinical Solutions market refers to the utilization of technology in the clinical trial process. This includes various electronic systems and software that aid in the management, analysis, and reporting of data from clinical trials. The use of eClinical solutions allows for more efficient and accurate data collection, ultimately leading to better decision-making regarding drug development.
Global eClinical Solutions Market size is expected to be worth around USD 27 Billion by 2032 from USD 8 Billion in 2022, growing at a CAGR of 12.8% during the forecast period from 2022 to 2032.
The market for eClinical solutions has experienced significant growth in recent years due to an increase in clinical trials being conducted globally. Factors such as rising R&D costs and growing demand for personalized medicine have also contributed to this growth. The market is expected to continue expanding as pharmaceutical companies increasingly adopt digital technologies.
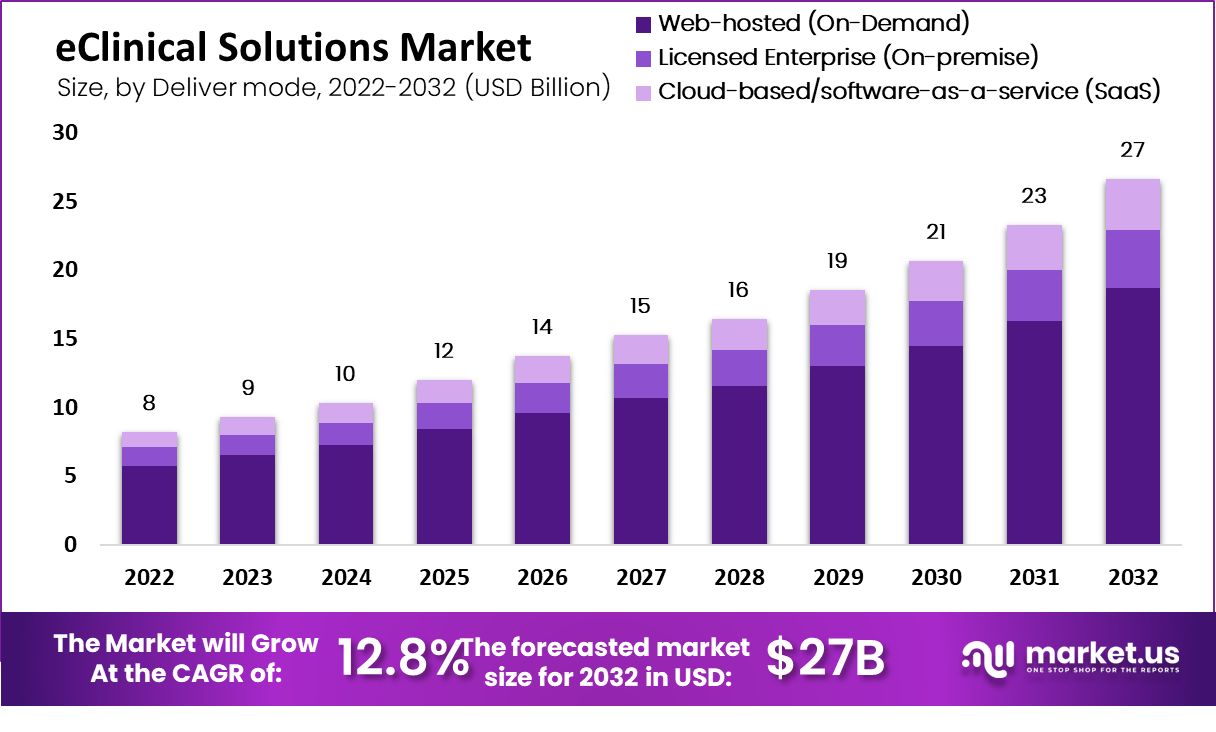
Key Takeaway
- By Product, in 2022, Electronic Clinical Outcome Assessment (eCOA) dominate the eClinical market.
- By Mode Of Delivery, Due to the higher level of interoperability of these products, the web-hosted sector, with a market share of USD $2973.24 million, dominated the market in 2022.
- By Development Phase, the phase III sector of clinical trials dominated the eClinical solutions market in 2022, as more pharmaceuticals successfully advanced to phase III.
- By end-user, the Pharmaceutical & Biotechnology Companies dominate the market.
- In 2022, North America dominated the market with the highest revenue share.
Request For Sample Report Here: https://market.us/report/eclinical-solutions-market/equest-sample/
Regional Snapshot
- North America: North America especially that of the United States, has been an important market for eClinical solutions. It has an established infrastructure for healthcare, an active scientific research and clinical sector, and an environment that is favorable for regulation. There are major biotech and pharmaceutical firms and contract research organizations (CROs) and research institutes of academic standing contributing to the development of the eClinical market for solutions in the region.
- Europe: Europe is another significant market that is a major one for eClinical solutions. States like Europe, the United Kingdom, Germany, France, and Switzerland are home to a large number of clinical research organizations and research centers of academic institutions. They are represented by the European Medicines Agency (EMA) has guidelines and regulations which determine the acceptance of eClinical solutions. There are also initiatives that promote the digitalization of the field of healthcare. This further boosts the marketplace.
- Asia Pacific: The Asia Pacific region has seen an increase in the growth of the electronic clinical solutions market. States like China, Japan, India as well as South Korea have been increasing their research focus and are rapidly becoming major actors in the pharmaceutical sector. A large population of patients and the increasing outsourcing of clinical research and investment in health infrastructure are major factors in the growth of markets within the Asia Pacific.
- Latin America: Latin America is a growing area in eClinical solutions. Countries like Brazil, Mexico, and Argentina are experiencing rapid growth in the manufacturing of pharmaceuticals and increasing trials. These regions offer cost benefits when conducting clinical research, and attract international sponsors as well as CROs. The government's efforts to simplify the regulatory process and improve capacity for clinical research are also driving the development of eClinical technology within the region.
- Middle East and Africa: The Middle East and Africa region is also a source of opportunities for the field of eClinical Solutions. Some countries such as Saudi Arabia, the United Arab Emirates, and South Africa have been investing in infrastructure for healthcare as well as capacities for clinical research. Participation in international clinical trials as well as attempts to meet international standards for regulatory compliance contribute to growth in the market.
Drivers
- Increasing Clinical Trial Complexity: The growing complexity of clinical trials, including larger sample sizes, multiple study sites, and diverse data sources, creates a need for advanced eClinical solutions to manage and analyze data efficiently. These solutions enable streamlined data collection, real-time monitoring, and improved trial management, leading to increased adoption.
- Demand for Efficient Data Management: The need to effectively manage and analyze the vast amount of data generated during clinical trials is a major driver of the eClinical solutions market. Electronic data capture (EDC) systems, clinical data management systems (CDMS), and an electronic trial master file (eTMF) systems offer centralized data storage, data validation, and real-time access to facilitate accurate and efficient data management.
- Regulatory Compliance and Data Integrity: The regulatory requirements for clinical trials, such as Good Clinical Practice (GCP) guidelines and data integrity standards, drive the adoption of eClinical solutions. These solutions help ensure compliance, improve data accuracy, enhance data security, and facilitate audit trails, thereby minimizing the risk of regulatory non-compliance.
- Technological Advancements: Technological advancements, such as cloud computing, mobile applications, artificial intelligence (AI), and machine learning (ML), have significantly influenced the eClinical solutions market. These advancements enable remote data collection, real-time monitoring, predictive analytics, and automation, leading to improved efficiency and productivity in clinical trials.
Restraints
- Data Security and Privacy Concerns: The sensitive nature of clinical trial data raises concerns regarding data security and privacy. Protecting patient data and maintaining confidentiality throughout the trial process is a critical challenge for eClinical solution providers. Stricter regulations, such as the General Data Protection Regulation (GDPR), impose compliance requirements and may restrict data sharing and accessibility.
- Integration Challenges: Integrating eClinical solutions with existing clinical trial systems and processes can be complex. Interoperability issues, data standardization, and compatibility with legacy systems pose challenges for seamless integration and data exchange, hindering the widespread adoption of eClinical solutions.
Opportunities
- Increasing Outsourcing of Clinical Trials: The growing trend of outsourcing clinical trials to contract research organizations (CROs) and emerging markets creates opportunities for eClinical solution providers. CROs often leverage eClinical solutions to enhance efficiency, reduce costs, and ensure data quality, driving the demand for these solutions.
- Rising Focus on Patient-Centric Trials: There is a growing emphasis on patient-centric clinical trials, which aim to improve patient experience, engagement, and participation. eClinical solutions that enable electronic patient-reported outcomes (ePRO) and patient recruitment platforms can enhance patient engagement and contribute to the success of patient-centric trials.
Make an inquiry before picking up this report @ https://market.us/report/eclinical-solutions-market/#inquiry
Challenges
- Cost and Budget Constraints: The implementation and maintenance costs of eClinical solutions can be a challenge for some organizations, particularly smaller research institutions and emerging markets with limited financial resources. Cost considerations and budget constraints may delay or limit the adoption of these solutions.
- Resistance to Change: The adoption of eClinical solutions often requires a shift from traditional paper-based processes to electronic systems. Resistance to change from stakeholders, including researchers, investigators, and study coordinators, can hinder the adoption and utilization of eClinical solutions.
- Training and User Acceptance: Effective training and user acceptance of eClinical solutions are essential for their successful implementation. User training and support are necessary to ensure that clinical trial personnel can effectively utilize the software and maximize its benefits. User-friendly interfaces and intuitive designs can help overcome user adoption challenges.
- Variability in Regulatory Frameworks: The variability in regulatory frameworks across different regions and countries presents challenges for eClinical solution providers. Adapting to diverse regulatory requirements and ensuring compliance with regional guidelines can be complex, especially for multinational clinical trials.
Key Market Players
- Oracle Corporation
- Medidata Solutions, Inc.
- Parexel International Corporation
- BioClinica, Inc.
- Signing Health
- Datatrak International, Inc.
- ERT
- eClinical Solutions, Inc.
- MaxisIT Inc.
- Bio-Optronics, Inc.
- Dassault Systemes
- IBM Watson Health
- Anju Life Sciences Software
- Merge Healthcare Incorporated
- OmniComm System
- Other Key Players
Key Market Segments
Product
- Electronic Data Capture (EDC)
- Clinical Data Management Systems (CDMS)
- Clinical Trial Management Systems (CTMS)
- Electronic Clinical Outcome Assessment (eCOA)
- Randomization and Trial Supply Management Solution (RTMS)
- Safety Solutions
- Analytics and Reporting Platforms
- Integration Platforms
- Electronic Trial Master File (eTMF)
Delivery Mode
- Web-hosted (On-Demand)
- Licensed Enterprise (On-premise)
- Cloud-based/software-as-a-service (SaaS)
Development Phase
- Phase I
- Phase II
- Phase III
- Phase IV
End-User
- Academic Institutes
- Medical Device Manufactures
- Hospitals
- CROs
- Pharmaceutical & Biotechnology Companies
Top Impacting Factors
- Increasing Clinical Trial Complexity: The rising complexity of clinical trials, including larger sample sizes, multiple study sites, and diverse data sources, drives the need for advanced eClinical solutions. These solutions help streamline and automate various aspects of clinical trial management, such as data collection, monitoring, analysis, and regulatory compliance.
- Technological Advancements: Advances in technology play a crucial role in shaping the eClinical solutions market. Innovations in cloud computing, mobile applications, artificial intelligence (AI), machine learning (ML), and big data analytics enable enhanced data management, real-time monitoring, predictive analytics, and automation, leading to improved efficiency and effectiveness of clinical trials.
- Regulatory Compliance and Data Integrity: Regulatory requirements and guidelines for clinical trials, such as Good Clinical Practice (GCP) and data integrity standards, have a significant impact on the eClinical solutions market. Compliance with these regulations drives the adoption of eClinical solutions that ensure data integrity, security, and compliance with regulatory standards.
- Growing Demand for Efficient Data Management: The increasing volume of data generated in clinical trials necessitates efficient data management solutions. eClinical solutions, including electronic data capture (EDC), clinical data management systems (CDMS), and electronic trial master file (eTMF) systems, provide centralized data storage, data validation, real-time access, and improved data quality and accuracy.
- Cost and Time Efficiency: eClinical solutions offer potential cost and time savings in clinical trial operations. They streamline processes, reduce manual errors, enable remote data collection, and enhance collaboration among stakeholders, leading to cost-effective and faster clinical trial execution.
Recent Developments
- With the introduction of SmartSignalsTM eConsent in 2021, Signiant Health expanded its electronic informed consent options and capabilities. Sponsors now have more control over getting electronic informed consent and re-consent for any research design due to changes in key product features and tiered licensing options. The most recent iteration of the Bright Clinical Data CloudTM, which was released in 2021 by eClinical Solutions LLC, delivers stronger analytics, more visualizations, and enhanced automation of data review and mapping capabilities across the platform.
Report Scope
| Report Attribute | Details |
| The market size value in 2022 | USD 8 Bn |
| Revenue Forecast by 2032 | USD 27 Bn |
| Growth Rate | CAGR Of 12.8% |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, and Middle East & Africa, and Rest of the World |
| Historical Years | 2017-2022 |
| Base Year | 2022 |
| Estimated Year | 2023 |
| Short-Term Projection Year | 2028 |
| Long-Term Projected Year | 2032 |
Frequently Asked Questions
Q: What is the current size of the eClinical Solutions Market?
A: The Global eClinical Solutions Market size is USD 8 Billion in 2022.
Q: What is the projected growth rate for eClinical Solutions Market?
A: The eClinical Solutions Market is expected to grow at a CAGR of 12.8% from 2023 to 2032.
Q: What are some of the key players in the eClinical Solutions Market?
A: Some of the key players in the eClinical solutions market include Oracle Corporation, Medidata Solutions, Inc., Parexel International Corporation, BioClinica, Inc., Signing Health, Datatrak International, Inc., ERT, eClinical Solutions, Inc., MaxisIT Inc., Bio-Optronics, Inc., Dassault Systemes, IBM Watson Health, Anju Life Sciences Software, Merge Healthcare Incorporated, OmniComm System, Other Key Players.
Contact
Global Business Development Team – Market.us
Market.us (Powered by Prudour Pvt. Ltd.)
Send Email: [email protected]
Address: 420 Lexington Avenue, Suite 300 New York City, NY 10170, United States
Tel: +1 718 618 4351
Website: https://market.us
Content has been published via 11press. for more details please contact at [email protected]
The team behind market.us, marketresearch.biz, market.biz and more. Our purpose is to keep our customers ahead of the game with regard to the markets. They may fluctuate up or down, but we will help you to stay ahead of the curve in these market fluctuations. Our consistent growth and ability to deliver in-depth analyses and market insight has engaged genuine market players. They have faith in us to offer the data and information they require to make balanced and decisive marketing decisions.